HyperMAG®
A new process produces nanoparticles ideal for magnetic hyperthermia.
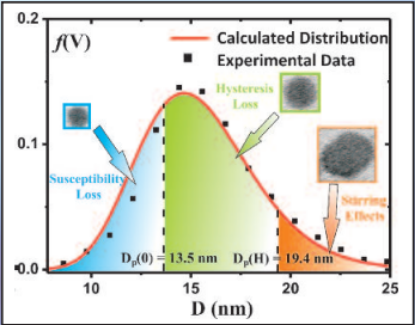
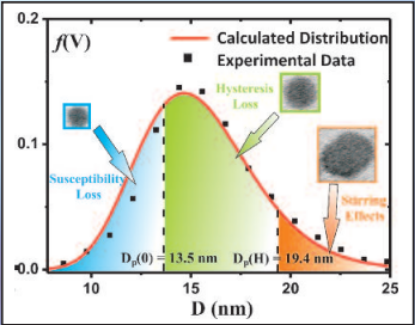
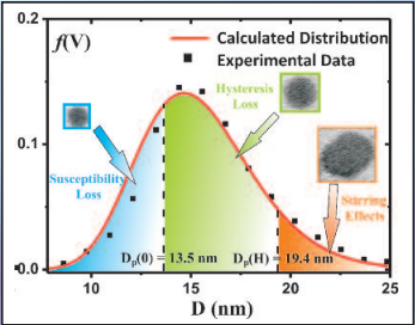
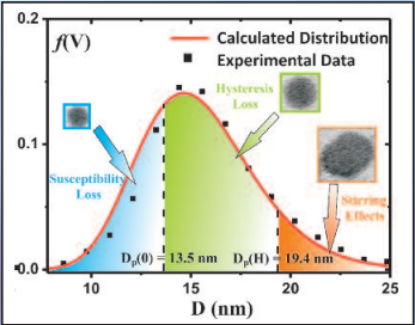
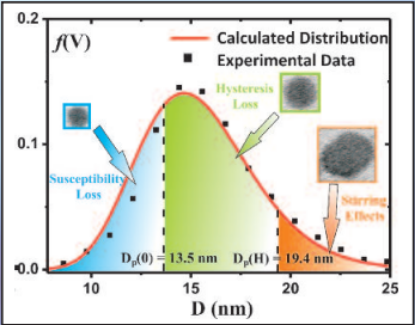
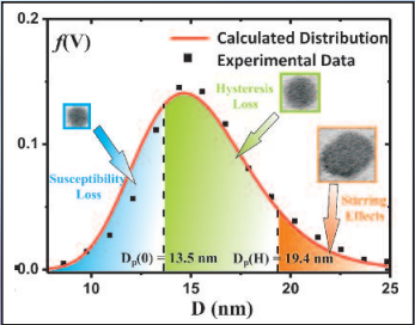
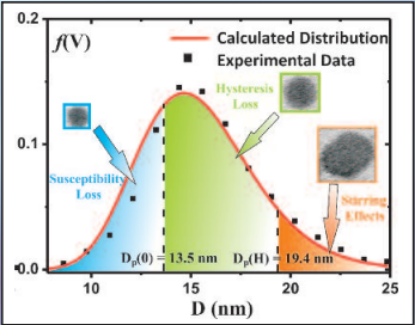
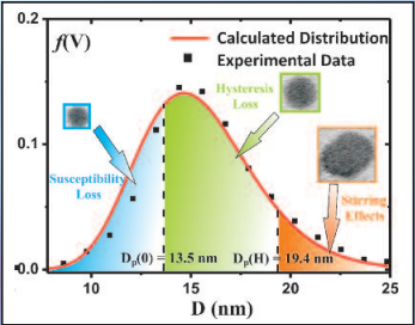
Three mechanisms contribute to magnetic hyperthermia
- susceptibility loss
- hysteresis loss
- viscous heating (stirring)
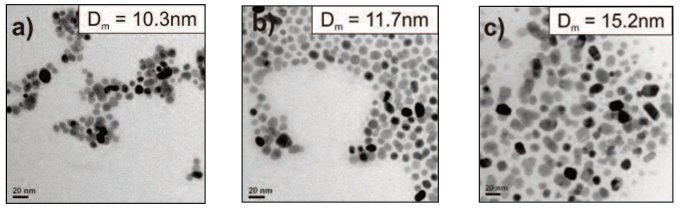
HyperMAG® particles are available with median diameters of 10.3, 11.7 and 15.2 nm
Particles are non-toxic due to an aqueous process for production.
In the diagram the size ranges for the three mechanisms are shown.
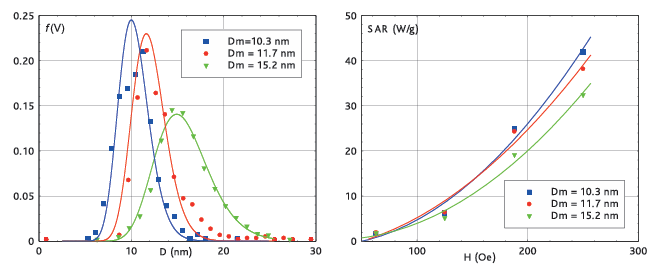
| Product; | Coresize(nm) | Hydrodynamicsize(nm) | Zeta-potential(mV) | Feconc.(mg/ml) | Solvent |
|---|---|---|---|---|---|
| HyperMAG®A | 10.3 | 100 | -40 | 100-40 | dH2O |
| HyperMAG®B | 11.7 | 100 | -40 | 100-40 | dH2O |
| HyperMAG®C | 15.2 | 100 | -40 | 100-40 | dH2O |
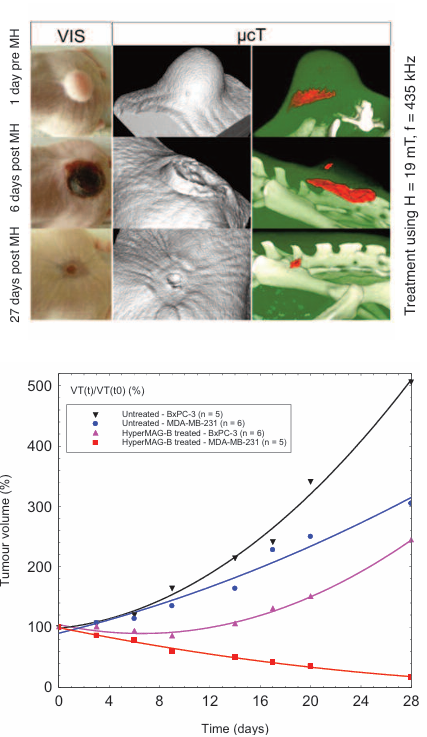
Treatment of breast and pancreatic cancer using HyperMAG-B
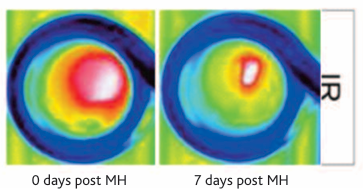
The images below show the treatment sequence for magnetic hyperthermia (MH) using HyperMAG-B. The external temperature of the tumour surface is shown on the right (IR) and the effect on tumour volume over a period of 27 days on the left (VIS). The intratumoural distribution of HyperMAG-B as determined by micro computed tomography, 24 hours prior to first hyperthermia treatment is also shown (μcT).
Conclusions of In-Vivo testing
- Magnetic hyperthermia using HyperMAG-B leads to effective tumour growth reduction.
- HyperMAG-B display a high heating potential at low dosage, ideal for in-vivo applications.
- Excellent biocompatibility of HyperMAG-B and localised therapy is tolerable for animals.
- Customised particles can be produced for special applications.
- Hyperthermia using HyperMAG-B results in reduced tumour volume in both cancer models.
- MDA-MB-231 tumour volumes were reduced to 50% (p≤0.01) over a 4 week period.
- BxPC-3 tumours were reduced in volume by 40% ( p≤0.05) over a 4 week period relative to untreated tumours.
- HyperMAG-B nanoparticles do not alter the blood composition after hyperthermia treatment.